현재 Idiopathy pulmonary fibrosis (IPF)에 관한 약물은 Pirfenidone과 Nintedanib밖에 없는 상황입니다. 하지만 pirfenidone은 3개 임상 3상을 통해 mortality 개선 효과를 봤지만, 위장관 부작용이 퍼페니돈 사용에 어려움을 주고 있습니다. 닌테다닙은 FVC와 급성악화, SGRQ에 대한 개선 효과를 보였지만 아직까지 mortality 개선에 영향을 못 보주고 있습니다. 이는 장기간 연구를 좀 더 지켜보면 더 좋은 효과가 나올 수 있습니다. 국내에서는 아직 닌테다닙에 급여가 적용되지 않아서 국내에서 사용하기엔 경제적인 부담이 있습니다. 이들 약물이 그동안 어려운 IPF 치료제 개발의 길을 닦았고, 다양한 약물이 IPF 치료제로 개발되고 있습니다. 국내에도 몇 가지 약물이 개발 중입니다.
아래 약물에 대한 그동안 기사, IR 자료에 대해서 자료를 찾아봤습니다. 내용이 없는 것도 있으니 양해 바랍니다.
대웅: DWN12088
브릿지바이오: BBT-877
나이벡: NIPEP-PF (NP-201)
한미: HM15211
1. 대웅 제약 DWN12088
1) 기사 내용
- Prolyl-tRNA synthetase (PRS) 작용을 억제-> 콜라겐 생성 억제
- 안전성과 효능 (FVC수치 악화 개선) 평가 계획
- FDA fast track
- 1상에서 162명 건강인, 안전성 확인
- 동물모델에서 기존 치료제보다 탁월한 효과를 확인했다.
2) IR 자료
- 작용기전: Prolyl-tRNA synthetase inhibitor (PRS inhibitor)
- 적응증 : IPF, 피부 경화증, 심장 섬유증, NASH 등
*Highlight*
Novel Target: add-on therapy potential with various agents
Efficacy: Superior anti-fibrotic effects in mouse pulmonary fibrosis model
Safety: Confirmed wider safety margin compared to current standard of care (Esbriet, Ofev)

3) Abstract
[Synergistic Anti-Fibrotic Effect of a First-in-Class PRS Inhibitor, DWN12088, and Standard-of-Care Therapeutic Agents for IPF]
Introduction:
Glutamyl-Prolyl-tRNA synthetase (EPRS) is an enzyme that conjugates proline to its tRNA. Excessive deposition of collagen is the pathological hallmark of fibrosis, and proline is one of the major constituents of collagen. Hence, dysregulation of EPRS could drive excessive collagen formation. Daewoong is developing a novel, proprietary first-in-class prolyl-tRNA synthetase (PRS) inhibitor, DWN12088, and previous studies have shown anti-fibrotic effects by down-regulating collagen synthesis in various cellular and mouse pulmonary fibrosis models. Currently, Pirfenidone and Nintedanib serve as standard-of-care (SoC) therapies for Idiopathic Pulmonary Fibrosis (IPF), yet the treatment effects are limited. Thus, we investigated potential synergistic anti-fibrotic effects of DWN12088 in addition to SoC.
Method:
Diseased human lung fibroblasts (DHLF) were co-treated with TGF-β1, DWN12088 and Nintedanib for 48 hr, then α-smooth muscle actin (α- SMA) and Collagen 1A1 (COL1 A1) protein levels were assessed by western blot.
The anti-fibrotic effect of DWN12088 and Pirfenidone co-treatment were investigated using Bleomycin (BLM)-induced mouse pulmonary fibrosis model. Mouse lung function was measured using pulse oximetry.
Results:
Using dose-range finding study, the effective target concentration that can down-regulate approximately 50% of the α-SMA and COL1A1 were identified as 5 μM DWN12088 and 50 nM Nintedanib. Cells were co-treated with 50nM Nintedanib and titration of DWN12088, dose-dependent reduction of α-SMA and COL1A1 were observed. Cells were co-treated with 5 μM DWN12088 and titration of Nintedanib, dose-dependent reduction of α-SMA and COL1A1 were observed as well. Finally, co-treatment of 50 nM Nintedanib and 5 μM DWN12088 inhibited α-SMA and COL1 A1 better than 100 nM Nintedanib or 10 μM DWN12088 alone.
In BLM-induced mouse pulmonary fibrosis model, co-treatment of DWN12088 and Pirfenidone significantly reduced total collagen amount and fibrotic index in the mouse lung tissues compared to treatment of each compound alone. Moreover, co-treatment of DWN12088 and Pirfenidone significantly improved lung function, as shown by pulse oximetry, compared to treatment of each compound alone.
Conclusion:
Current standard of care for IPF, Pirfenidone and Nintedanib, show limited therapeutic effects in patients. Daewoong’s proprietary PRS inhibitor, DWN12088, has anti-fibrotic effects by inhibiting collagen synthesis and down-regulating pro-fibrotic markers. When combined with either Pirfenidone or Nintedanib, DWN12088 exhibits synergistic anti-fibrotic effect in both in vitro and in vivo IPF models.
This suggests that DWN12088 has potential to become a novel therapeutic agent for IPF, either as monotherapy or combination therapy. DWN12088 has completed Phase I study in healthy volunteers, and is expected to enter Phase II clinical trial in 2021.
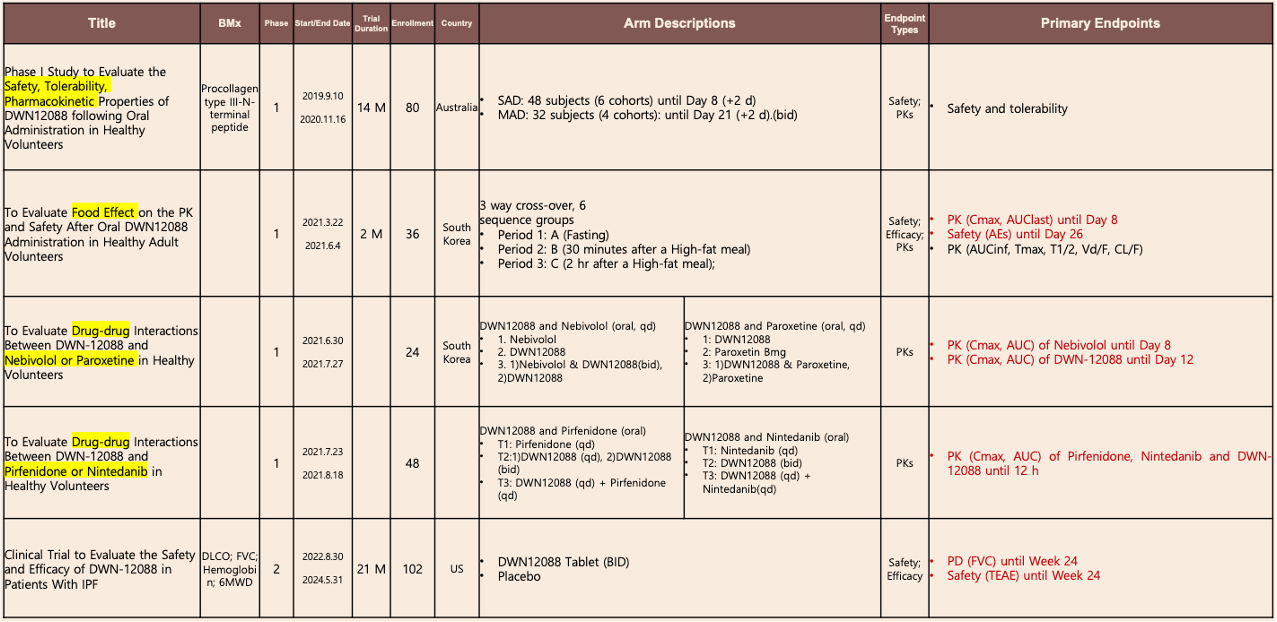
2. Clinical Trials
'심각한_질병이야기 > Respiratory disorder' 카테고리의 다른 글
| 닌테다닙(Nintedanib) 개발 상황 리뷰 (0) | 2022.09.22 |
|---|---|
| 브릿지바이오 BBT-877 [국내 IPF 치료 약물 개발 2] (0) | 2022.09.21 |
| 현재까지 알려진 COPD biomarker 모음 (0) | 2022.09.15 |
| 특발성 폐 섬유증과 방사성 폐렴의 차이 (IPF vs Radiation pneumonitis) (0) | 2022.09.14 |
| Pamrevlumab(FG-3019)_특발성 폐 섬유증(IPF) 치료제의 연구내용 (0) | 2022.07.02 |